Current evidence about the safety of COVID-19 vaccines relies mainly on data from phase 1–3 randomized controlled trials and vaccine safety surveillance system in several countries. We found three reviews of the safety of COVID-19 vaccines , which combined study experimental groups, and did not examine the heterogeneity between vaccine platforms and participant age groups. Here, we conduct a rapid review and meta-analysis to summarize the safety data of COVID-19 vaccine candidates.
We aim to comprehensively evaluate the rate of solicited, unsolicited, and serious AEFI in each clinical trial and to estimate the relative risk of AEFI by vaccine platform and participant age group. We also collected post-authorization surveillance data from around the world to look for uncommon and delayed onset reactions. This overview of the safety profile of COVID-19 vaccines will support responses to potential safety issues and inform decision-makers evaluating vaccination strategies around the globe. The pooled rates of local and systemic reactions were significantly different between vaccine platforms.
Inactivated vaccines, protein subunit vaccines, and DNA vaccines had lower rates of local and systemic reactions compared to RNA vaccines, non-replicating vector vaccines, and virus-like particle vaccines. The safety profiles of BNT162b2, mRNA-1273, ChAdOx1-nCoV, Ad26.COV2.S, and CoronaVac were relatively benign in the elderly, and both the frequency and the intensity of local and systemic reactions decreased with age. The rates of SAE, including non-fatal serious AEFI and death, were similar in vaccine and placebo groups in clinical trials. Reporting rates of common AEFI after mass public vaccination were lower than in clinical trials.
Several unexpected rare adverse events, which resulted in severe outcomes, have been noted in post-authorization surveillance. As of May 9, 2021, about 0.6 billion people around the world had been vaccinated with at least one dose of COVID-19 vaccines, accounting for about 7.8% of the world's population . This mass vaccination should allow for the identification of more uncommon and rare AEFI. According to the Vaccine Adverse Event Reporting System and V-safe system of the US Centers for Disease Control and Prevention , the rates of non-serious AEFI after public administration of BNT162b2 and mRNA-1273 were similar to the clinical trials.
Variations in the incidence of anaphylaxis between countries are to be expected, as the numbers vaccinated in most countries to date are relatively small compared with the USA, and the reporting rates of AEFI from passive surveillance are biased. A causal link of thrombosis and thrombocytopenia with adenoviral vector vaccines (ChAdOx1 nCoV-19 and Ad26.COV2.S) was noted after mass public vaccination, including several deaths and severe outcomes . While rare side effects should not derail vaccination efforts , a thorough risk-benefit analysis is required. Several studies have explored the safety profile of two mRNA vaccines (BNT162b2 and mRNA-1273) in HIV-positive populations , immunosuppressive patients , and pregnant women , revealing no evidence of unexpected serious adverse events.
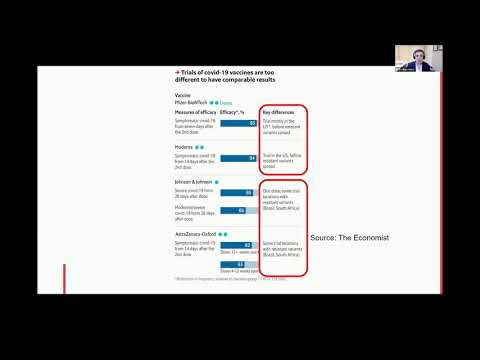
Further evaluation of the benefit-risk profile is warranted in these specific populations. Differences in safety profiles of vaccines must be considered in the context of efficacy. Both RNA vaccines (BNT162b2 and mRNA-1273) reported 95% and 94% vaccine efficacy, respectively (symptomatic PCR-confirmed cases were the primary clinical trial outcomes). This is substantially higher than the reported efficacy of other vaccine platforms.
The efficacy of inactivated vaccines was reported as 78.1% for BBIBP-CorV and 50.7% for CoronaVac . Efficacy of Ad26.COV2.S against moderate to severe critical Covid-19 with onset at least 14 days after administration was 66.9% . Overall efficacy of ChAdOx1-nCoV in preventing symptomatic COVID-19 across both the low dose and standard dose groups was reported as 70.4% .
The efficacy of Gam-COVID-Vac, another non-replicating vector vaccine, was 91.6% . Based on the current evidence, RNA vaccines have both higher rates of adverse reactions and higher efficacy. Due to the relative mild and transient nature of most of these reactions, RNA vaccines should be considered an excellent option to protect against COVID-19, especially in the absence of other viable candidates with similar efficacy.
In addition to safety and efficacy, vaccine candidates must also be assessed in the context of the risk of disease, to determine whether each vaccine supports a favorable benefit-risk ratio or not. Such a determination is undoubtedly more important than comparing safety and efficacy between vaccine candidates as long as vaccine supply is limited and disease is prevalent. These platforms can be classified either as traditional approaches that have previously resulted in licensed vaccines (e.g., inactivated, recombinant proteins, vectored vaccines), or as approaches that have never before been used for a licensed vaccine (e.g., RNA and DNA vaccines) . Common reasons given for not intending to receive these vaccines included "concern about the safety of the vaccine in its development" and "potential side effects" . As mass vaccination has progressed, more occurrences of adverse events following immunization have been reported, especially the rare AEFIs. Demonstrating and summarizing vaccine safety from clinical trials and post-authorization surveillance is critical for public confidence, and for enabling timely, evidence-based policy decisions for population-level use .
If data for the same subjects were presented in multiple publications, these data were only counted once. Due to phase 1 and 2 trials often including multiple differing experimental groups, we focused exclusively on safety data from experimental groups in phase 3 clinical trials. Any discrepancies were resolved by consensus or in consultation with a third researcher.
Two researchers (Q.W., X.C.) assessed the methodological quality of studies using the Cochrane risk of bias tool . Certainty of evidence was assessed by researchers according to the grading recommendations assessment, development and evaluation framework . In conclusion, the available evidence indicates that eligible COVID-19 vaccines have an acceptable short-term safety profile.
Additional studies and long-term population-level surveillance are strongly encouraged to further augment the safety profile of COVID-19 vaccines. All reports of suspected adverse reactions should be investigated and warning signals rapidly evaluated, to allow implementation of appropriate risk minimization measures and update the benefit/risk ratio of vaccination. The safety profiles of COVID-19 vaccines are still incomplete, even for those currently in use. The safety and efficacy of COVID-19 vaccines in certain subpopulations, such as children and adolescents, pregnant woman, and people with multiple underlying conditions, have not yet been fully studied.
Although crude reporting rates of AEFIs from post-authorization safety monitoring have so far been lower than in clinical trials, adverse reactions that are uncommon or have delayed onset require extended post-authorization study to detect. Investigation of safety signals, a lack of epidemiological tools for active surveillance, obstacles at the national regulatory authority level, and a lack of information sharing between countries are still major challenges for most countries. Pharmacovigilance mechanisms must be put in place, with all the necessary training, especially in low- and middle-income countries .
Further study will strengthen and expand upon our knowledge in these areas and enable the refinement of vaccine recommendations and injury compensation programs. Safety issues noted in mass vaccination may have a deleterious impact on the global vaccine supply and the already fragile confidence in vaccines. Government agencies and vaccine developers should continue to take action to encourage vaccination and reduce public vaccine hesitancy.
Our search identified a total of 7231 records after removal of duplicates (Fig.1). After initial title/abstract screening, 157 articles were assessed for eligibility via full-text review. For the safety data among general population, 43 articles reporting on 19 vaccines of 6 different platforms and 10 documents released by WHO , US Food and Drug Administration and UK Medicines & Healthcare products Regulatory Agency from clinical trials were included. A total of 123,540 study participants receiving COVID-19 vaccines and 97,944 participants receiving placebos were included in safety set of clinical trials.
Post-authorization safety profiles were assessed through 3 reports released by the European Medicines Agency , 20 reports including large-scale monitoring data , 11 observational studies , and 26 reports from countries' national surveillance systems. For the safety profile of COVID-19 vaccines in clinical trials, the primary outcomes were the proportion of vaccine recipients experiencing at least one AEFI and the rates of selected AEFI of COVID-19 candidate vaccines versus placebos. We specified severe versus mild-to-moderate AEFI in our extraction and analyzed these categories separately. For post-authorization safety data, we examined rates of AEFIs, serious adverse events , and adverse events of special interest .
We explored the reasons for variations among eligible vaccines and examined whether rate of AEFI varied by vaccine platform, age group of participants and serostatus of participants against SARS-CoV-2 at baseline. For the purposes of stratifying safety data by age group, we defined younger adults as under 65 years of age and elderly as over 65 years of age. If the age group of the clinical trial was not completely consistent with our study, the safety data of the closest age stratification was extracted.
We classified all participants in the Ad5 nCoV trials as younger adults, since no stratified analyses by age were performed and the proportion of the participants under 55 years old was reported as 86% . English-language articles and results posted on PubMed, Embase, Web of Science, PMC, official regulatory websites, and post-authorization safety surveillance data were searched through June 12, 2021. Publications disclosing safety data of COVID-19 candidate vaccines in humans were included. A meta-analysis of proportions was performed to estimate the pooled incidence and the pooled rate ratio of safety outcomes of COVID-19 vaccines using different platforms. Firstly, we only included data reported at the study level, due to limited access to individual-level data.
Secondly, there are factors we did not include in the meta-analysis, such as seropositivity against SARS-CoV-2 at baseline and underlying conditions, so the potential effects of such heterogeneity were not quantitatively assessed. Thirdly, in the clinical trials for BNT162b2 and ChAdOx1-nCoV, age groups were divided at 55 years of age, which differed from our subgroup analysis of age divided at 65 years of age. Finally, although we included currently available post-authorization safety monitoring data, such monitoring programs are still in their infancy and often rely on a mix of active and passive surveillance. Three researchers (Q.W., X.C., X.B.) assessed eligible studies, conducted data extraction, and cross-checked. We looked for clinical trials and post-authorization reports that examined safety data of COVID-19 candidate vaccines, and included manuscripts published in peer-reviewed journals, preprints, and unpublished data disclosed by regulatory agencies. We excluded study protocols, media news, commentaries, reviews, case reports, reports of non-human clinical trials, reports among specific populations , and abstracts of congress meetings or conference proceedings.
We also excluded interim reports of clinical trials that did not clearly show safety data of specific COVID-19 candidate vaccines selected for further use, and reports on vaccines no longer under clinical evaluation. Post-authorization observational studies with sample sizes less than one thousand were excluded as well. Limitations of this study include the lack of data on cellular immunity and on neutralizing antibodies, as well as the specific focus on health care workers.
Whether the observed difference in antibody level translates to a difference in the duration of protection,4 the protection against variants of concern, and the risk of transmission6 needs further investigation. Future research should also address the relevance for patients with reduced antibody response after vaccination. Based on a random-effect meta-analysis model, we used the inverse variance method to estimate pooled rate by platform, and the Clopper-Pearson method to calculate 95% confidence intervals . Heterogeneity tests (chi-squared test) with Higgins' I2 statistics were used to determine the extent of variation between vaccines.
Multivariate meta-regression models were used to determine the relative contribution of vaccine platform and age of participants to the rate of AEFI. Small study effects were assessed using funnel plots, and formally tested through the rank correlation test for those meta-analyses including more than 10 studies. All statistical analyses were done using R (version 4.0.2), using the "meta" package to conduct the meta-analysis. For all statistical tests, two tailed P-value less than 0.05 were considered statistically significant. Safety data on Russian vaccines need to be disclosed further so that safety signals can be identified and appropriate risk minimization measures quickly implemented.
Of 2499 health care workers who received 2 doses of SARS-CoV-2 mRNA vaccines, 1647 participated in this study. A total of 688 were vaccinated with mRNA-1273 (mean age, 43.2 years; 76.7% women; 21.8% previously infected with SARS-CoV-2), and 959 with BNT162b2 (mean age, 44.7 years; 84.9% women; 13.2% previously infected). Direct comparisons between efficacy data should also be interpreted with caution due to the inconsistency of environmental risk, endpoints, and statistical methods between studies. Current efficacy data show that all authorized vaccines exceed the 50% threshold set by WHO , indicating they prevent substantial disease, especially severe cases. Authorized COVID-19 vaccines can prevent a large proportion of symptomatic cases, hospitalizations, severe diseases, and death . Available evidence indicates that eligible COVID-19 vaccines have an acceptable short-term safety profile.
Additional studies and long-term population-level surveillance are strongly encouraged to further define the safety profile of COVID-19 vaccines. The rapid process of research and development and lack of follow-up time post-vaccination aroused great public concern about the safety profile of COVID-19 vaccine candidates. To provide comprehensive overview of the safety profile of COVID-19 vaccines by using meta-analysis technique.
Commented on the data and its interpretation and revised the content critically. All authors contributed to review and revision and approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Health care workers at a tertiary care center (Ziekenhuis Oost-Limburg, Belgium) who were scheduled for vaccination with 2 doses of either mRNA-1273 or BNT162b2 were invited to participate in this prospective cohort.
Serologic testing was performed prior to vaccination as well as 6 to 10 weeks after the second dose . Total immunoglobulin levels to the receptor-binding domain of the SARS-CoV-2 spike protein were measured with an anti–SARS-CoV-2 S enzyme immunoassay . After vaccination, antibodies against the SARS-CoV-2 nucleocapsid protein were determined. Previous infection was defined as anti-nucleocapsid positivity at any point, anti-spike positivity before vaccination, and/or a history of positive polymerase chain reaction results on nasopharyngeal swab.
The SARS-CoV-2 messenger RNA vaccines BNT162b2 (Pfizer-BioNTech) and mRNA have each shown more than 90% efficacy in preventing COVID-19 illness1,2 but, to our knowledge, humoral immune responses have not been compared directly. Has received research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company, and Shanghai Roche Pharmaceutical Company. None of those research funding is related to development of COVID-19 vaccines.





















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.